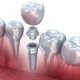
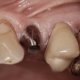
Dental Implants recommendations
Consensus Development Conference Statement
June 13-14, 1978
This statement is more than five years old and is provided solely for historical purposes. Due to the cumulative nature of medical research, new knowledge has inevitably accumulated in this subject area in the time since the statement was initially prepared. Thus some of the material is likely to be out of date, and at worst simply wrong. For reliable, current information on this and other health topics, we recommend consulting the National Institutes of Health's MedlinePlus .
This statement was originally published as: Dental Implants: Benefit and Risk. NIH Consens Statement 1978 Jun 13-14;1(3):13-19
For making bibliographic reference to the statement in the electronic form displayed here, it is recommended that the following format be used: Dental Implants: Benefit and Risk. NIH Consens Statement Online 1978 Jun 13-14 [cited year month day];1(3):13-19.Introduction
Guidelines for the use of dental implants were issued at the Harvard-National Institutes of Health-National Institute of Dental Research Consensus Development and Technology Assessment Conference held June 13-14, 1978, in Boston. Official statements and full proceedings will be published and available in early 1979. Dr. Paul Schnitman and Dr. Leonard Shulman, co-directors of the Harvard Tooth Implant-Transplant Research Unit at the School of Dental Medicine, organized, convened, and moderated the conference and have prepared this summary of the conference's objectives and recommendations.
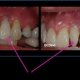
Much information regarding the safety and effectiveness of dental implants is controversial, presently existing as the result of individual experience and opinion rather than scientific fact. Although implant authors are prolific, extremes of success and failure are commonplace and these conflicting reports are difficult to interpret. Essentially, common criteria have not been used by implant authors to judge success and failure. Generally, conclusions have been based only on whether implant devices are or are not in place and whether or not they are symptomatic. Little attention has been given to data relating to true efficacy, e.g., is the implant standing on its own and truly helping the patient, or is it being held in place by the bridge it was designed to support? Is the patient left measurably worse off then before the procedure? Would the benefit/risk ratio of conventional dentistry have been better than that of implantation?
Clinically, thousands of patients have been treated with dental implants for years and there is no question that many have received long-term benefits. Some implants, on the other hand, fail in patients within six months; some have resulted in extensive bone loss and produced irreversible defects and complications.
Problems related to implant use frequently stem from poor patient selection. Insufficiently informed patients, and the unrestricted use of implants inadequately tested. These implant problems have led to a large body of unhappy patients and disappointed dentists.
In response to the concern of both the public and the profession, the American Dental Association and the Food and Drug Administration have taken steps to assure that these devices be reasonably safe and effective. The ADA has developed an implant registry to establish uniform case reports in the hope that in three to five years reasonably reliable information on safety and efficacy will be available. The FDA has now projected standards, classifications and limitations on all medical devices, including dental implants.
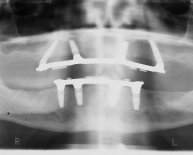
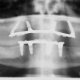
The purpose of this conference was to provide the dental consumer, practitioner, and researcher with a balanced and realistic appraisal of the four most commonly used implants in current clinical practice; the subperiosteal; staple/transosteal; vitreous carbon; and blade.
The NIH has developed, in the past year, the concept of technology assessment/consensus development, and the first such conference was held in 1977 to assess mammography as a screening and diagnostic technique for breast cancer. The decision of the medical field to modify its guidelines for mammography was a major outcome of the proceedings.
The NIH Office of the Medical Applications of Research (OMAR) has scheduled 20 consensus development meetings. This conference "Dental Implants: Benefit and Risk" was the third consensus conference and sought technical consensus on definitions of success, benefit and risk, and guidelines for use of the major dental implant types now available.
Paul Goldhaber, D.D.S., Dean of the Harvard School of Dental Medicine; Seymour Perry, M.D., Director of OMAR; David Scott, D.D.S., Director of NIDR; and David Link, Director of the Bureau of Medical Devices, Food and Drug Administration, outlined the importance and relevance of the conference to the field of dentistry, NIH, NIDR, and FDA.
In welcoming the group to Harvard. Dean Goldhaber said this conference was the first of what he hoped would be a series of real evaluations of what we do in the dental profession. He further quoted from an article by Frazier and Hiatt (Evaluation of medical priorities. Science, 200:875-878, 1978): "The term efficacy should be used to describe the net benefits and risks of an intervention. Both society and the individual have a stake in the measurement of efficacy with fair representation of the available information on risks and benefits to the individual that results from the intervention. Also, that as the largest single source of financial support for health care, society has a growing interest in the cost effectiveness of medical interventions."